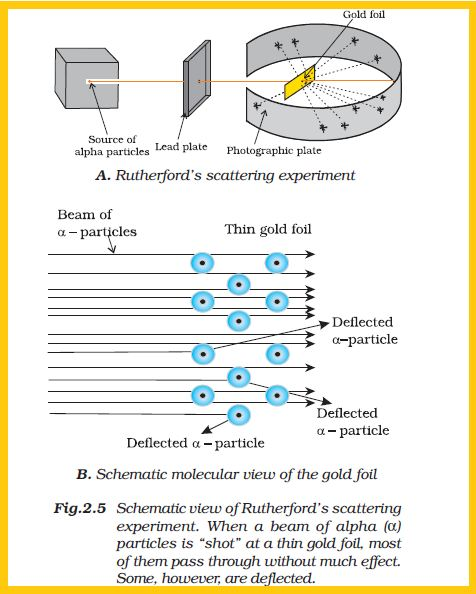
Atomic Models :
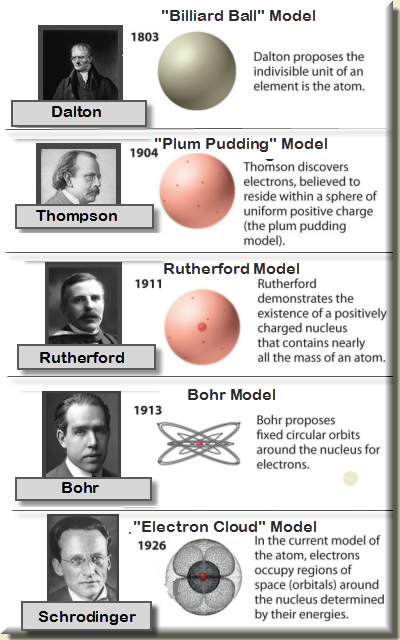
Existence of electron, proton and neutron suggested that Dalton’s indivisible atom is composed of sub-atomic particles.
Various atomic models were proposed to explain the distributions of these charged particles in an atom. Some of these models couldn't explain the stability of atoms.
Various atomic models were proposed to explain the distributions of these charged particles in an atom. Some of these models couldn't explain the stability of atoms.